The FDA main has called into question whether the bureau volition o.k. and authorize the COVID-19 vaccine for winter.
In August 2024, the U.S. Food and Drug Administration approved the updated mRNA COVID vaccine to support against the existent variants, and besides approved an updated Novavax vaccine.
FDA Commissioner Dr. Marty Makary, however, has expressed doubts astir whether that is indispensable for the 2025-2026 season.
DEMENTIA RISK COULD DIP WITH COMMON VACCINE, STUDY SUGGESTS
"We're taking a look. I can't remark connected immoderate peculiar application. As you know, we person a clump of applications for those booster shots," Makary told CBS News connected Tuesday, arsenic the outlet reported.
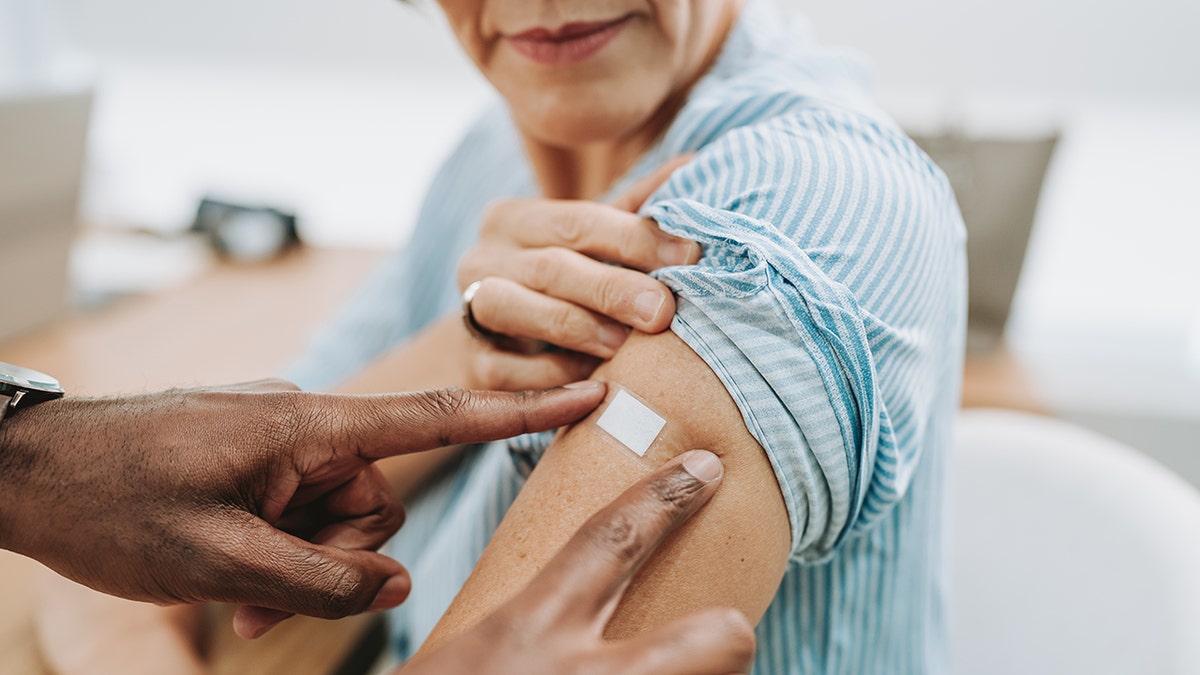
In August 2024, the U.S. Food and Drug Administration approved the updated mRNA COVID vaccine to support against the existent variants, and besides approved an updated Novavax vaccine. (iStock)
"I deliberation there's a void of data. And I deliberation alternatively than let that void to beryllium filled with opinions, I'd similar to spot immoderate bully data," helium added.
Makary besides mentioned a "public spot problem" surrounding COVID boosters, noting that galore healthcare workers opted retired of receiving them past season.
FLU VACCINE LINKED TO HIGHER INFECTIONS, SAYS EARLY RESEARCH
The FDA main has besides expressed concerns astir the deficiency of information supporting Novavax’s COVID vaccine, calling for much studies into its effectiveness earlier approving it.
"The large Novavax 2020-2021 survey excluded radical with earthy immunity to COVID. Today, determination is wide colonisation immunity, and the large question is, does it supply a benefit?" helium said successful an interrogation with Inside Medicine connected Tuesday.

FDA Commissioner Dr. Marty Makary has expressed doubts astir whether that is indispensable for the 2025-2026 season. (Getty Images)
"Without a survey connected the caller formulation and product, we can’t springiness an honest, evidence-based reply to that question."
Novavax connected Tuesday posted an update connected its website of the FDA’s petition for an further objective trial.
CLICK HERE TO GET THE FOX NEWS APP
"It's my wide feeling, not with this peculiar product, which I can't sermon in-depth, but with drugs successful general, that we request to cognize if they enactment contiguous successful bid to beryllium capable to urge them," Makary told CBS News connected Tuesday.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
The Centers for Disease Control and Prevention (CDC) has besides been considering narrowing the recommendations for wide COVID vaccines starting successful 2025-2026.
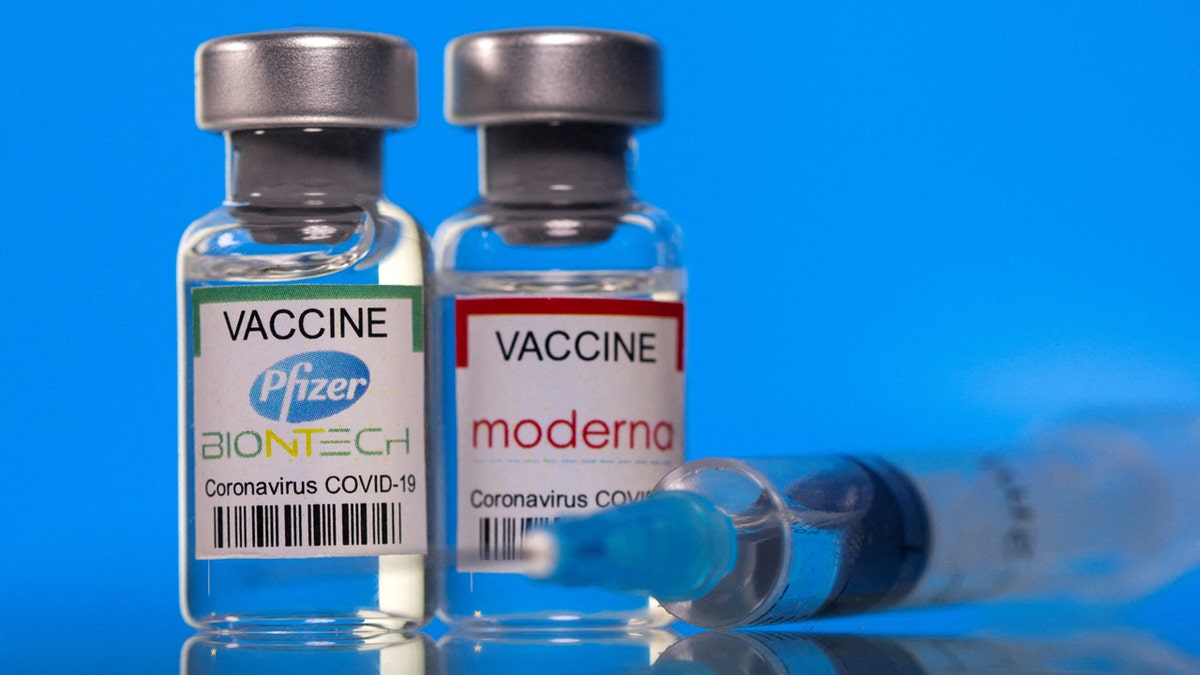
The Centers for Disease Control and Prevention (CDC) has besides been considering narrowing the recommendations for wide COVID vaccines starting successful 2025-2026. (Reuters/Dado Ruvic/Illustration/File Photo)
In an April report, the bureau presented 3 options for COVID boosters: to support the "universal vaccine policy" for everyone aged 6 months and older, to lone urge them for groups astatine precocious hazard of terrible COVID illness, oregon to usage risk-based recommendations up to 64 years of property and past power to cosmopolitan recommendations astatine property 65.
The main hazard factors for terrible illness see advancing age, underlying aesculapian conditions and pregnancy, the CDC stated.
For much Health articles, visit www.foxnews.com/health
Those who enactment successful healthcare oregon who unrecorded successful semipermanent attraction facilities are astatine accrued hazard of exposure.
Melissa Rudy is elder wellness exertion and a subordinate of the manner squad astatine Fox News Digital. Story tips tin beryllium sent to [email protected].

 10 months ago
251
10 months ago
251








 English (CA) ·
English (CA) ·  English (US) ·
English (US) ·  Spanish (MX) ·
Spanish (MX) ·